
Academic entrepreneurs looking to translate their research from the lab to the marketplace often face a daunting challenge: the valley of death.
This is the point in the innovation cycle where many technologies meet their demise. It represents the period when opportunities for governmental funding that support fundamental science breakthroughs have been exhausted, at the same time when industry investors are waiting for more testing and finishing touches.
For researchers at Arizona institutions, the Flinn Foundation provides a bridge across this difficult entrepreneurial passage. In 2021, the Flinn Foundation awarded seven $100,000 bioscience Translational Research Seed Grants, including two to researchers in the Ira A. Fulton Schools of Engineering at Arizona State University.
Jennifer Blain Christen, an associate professor of electrical engineering, is optimizing molecular analysis technology to bring fast and accurate COVID-19 diagnostics to remote locations. Jitendran Muthuswamy, an associate professor of biomedical engineering, is developing a noninvasive brain stimulation treatment for chronic migraines.
“The Flinn Foundation grant not only financially supports us so we can focus on final improvements, it provides training in how to take something to market,” Blain Christen said.
Increasing access to fast, reliable COVID-19 testing
Rapid COVID-19 testing has been an essential component of suppressing the spread of the pandemic. But without the right infrastructure, it can be nearly impossible to test quickly and effectively.
With the help of the Flinn Foundation grant, Blain Christen is refining the technology for a highly accurate handheld COVID-19 diagnostic device that provides quick results to support this vital service in underserved areas.

“The Flinn Foundation has been helping entrepreneurs and people in the health technology space for a very long time,” Blain Christen said. “That they think we have something and we’re on the right track is validating and helps us have some self-assurance that we have potential.”
Blain Christen has years of experience developing diagnostic systems with FlexBioTech, a startup she founded with her business partner, Dr. Karen Anderson, a medical oncologist and associate professor with Mayo Clinic and professor at the ASU Biodesign Institute.
She is also leveraging insights from the robust COVID-19 saliva testing process and infrastructure developed by the ASU Biodesign Clinical Testing Laboratory.
“We want to take the precision and skills that people in the ASU Biodesign Institute learned over many years and automate that so people using the test don’t need more than a YouTube video worth of training,” said Blain Christen, who is a Fulton Entrepreneurial Professor and faculty member in the School of Electrical, Computer and Energy Engineering, one of the six Fulton Schools.
Instead of delicate lenses, precisely aligned lasers and robotic test handling typical of other COVID-19 testing methods, Blain Christen’s device uses LEDs “smushed together like a sandwich” and other easily obtainable and inexpensive components that are portable and robust enough to survive bumpy rides on dirt roads to rural clinics.
The device works by shining an LED light onto a saliva sample to initiate “molecular amplification.” This process multiplies the amount of coronavirus particles in the sample through components in the testing vial and chemical reactions with heat. It’s like turning the volume up on your stereo, Blain Christen said.
The amplified coronavirus particles emit fluorescent light, which the diagnostic device detects to yield a positive result. The amplification step is critical to be able to detect early infections in which few virus particles are present in the body.
A laser can complete this process extremely quickly, but an LED can do so, too, for a fraction of the cost with the tradeoff of speed.
“We’re taking many, many measurements over time,” Blain Christen said. “It’s less accurate instantaneously, but if we measure over 30 seconds we can average out the inaccuracies. Engineers love averaging to increase accuracy.”
The unique device yields accurate results in about 40 minutes without expensive equipment or expertise. Because results are available so quickly, a person being tested is less likely to be exposed to the virus, or to spread it, between the testing time and results, which is the case when processing takes days or more.
Effective relief for the chronic migraine community
Chronic migraine affects more than 4 million people in the world through debilitating headaches that occur at least 15 days out of every month.
Few effective pharmacological treatments are available for this neurological condition. Even with promising new drugs in development, such treatments often have side effects that can affect the entire body. Additionally, many existing brain stimulation techniques are inconsistent, and those that work consistently are highly invasive with wires and batteries surgically implanted inside a person’s head, risking infection.
Muthuswamy, who focuses his research on use-inspired neuromodulation technologies, has been developing a targeted brain stimulation system to treat chronic prain that is noninvasive but matches the effectiveness of invasive technologies. The Flinn Foundation grant is helping him “de-risk” the technology — optimizing and validating it for use to attract investors.
“The Flinn Foundation’s support is unique,” said Muthuswamy, a faculty member in the School of Biological and Health Systems Engineering, one of the six Fulton Schools. “I have not had a grant where the expectation is entirely translational. The goal is to get it out of the lab and into the marketplace.
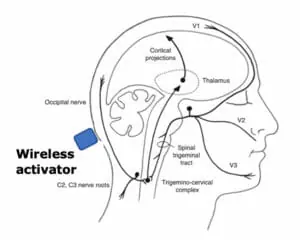
The technology involves what are called wireless injectable neurostimulation devices, or WINS, that are injected under the skin in the back of the head where the occipital nerve lies. Only a few millimeters in size, WINS do not have batteries or wires that break the skin, so they are much less invasive than other neurostimulation solutions.
A person experiencing migraines would activate the injected WINS devices with a handheld piece of equipment that emits sound waves. The WINS devices convert the sound waves into electricity, which is used to directly stimulate the occipital nerve and relieve the pain associated with chronic migraine.
Muthuswamy will work with co-inventor and Professor Emeritus Bruce Towe, the original inventor of the sound wave energy transfer technology. Muthuswamy is also working with neurosurgeon Dr. Zaman Mirzadeh at the Barrow Neurological Institute to ensure the device is effective.
“Dr. Mirzadeh will be involved every step of the way,” Muthuswamy said, “to make sure we’re putting the device in the right place, looking for the right responses and targeting the clinically relevant locations for injection.”
Without input from neurosurgeons who would use such a device, translating this research from lab to clinic can be “tricky,” Muthuswamy said.
“I can’t tell you the number of years I spent designing something that no neurosurgeon will use because it’s too small. They need something that they can handle and not break when they use it,” Muthuswamy said. “In the operating room setting, the context is very different (from the lab).”
If successful, Muthuswamy will have a technology targeted to treat migraine pain without potential side effects and that is usable on demand. With the guidance of the Flinn Foundation, he will be making crucial steps toward limited clinical trials in the next year or so.
“I’m excited to do this translational work and move down this path,” he said.
Resources beyond funding enable translational research
While the funding from the Flinn Foundation Translational Research Seed Grants is important to making translational progress, Muthuswamy and Blain Christen also find tremendous value in the networking opportunities the foundation provides.
“I see the Flinn Foundation as a hub for a lot of investors, innovators, clinicians and health care providers,” Muthuswamy said. “Besides funding, they provide a lot of opportunities for learning and networking.”
For Blain Christen, who knows the difficulty of finding locations and people to validate technologies with her startup, FlexBioTech, it’s like solving a puzzle without the picture on the box.
“The Flinn Foundation knows where all the pieces of the puzzle are, they know the whole picture,” she said. “They bring together all the knowledge of where all those resources are throughout the Valley that you need to make a successful business.”