
From head toe, we are a rich conglomerate of cells, constructed of and managed by proteins. Proteins are the building blocks of life, and the role they play in our bodies can determine whether we are healthy or sick. In 2018, researchers calculated that there are approximately 42 million protein molecules per cell.
Despite valiant efforts, we know very little about proteins. The problem is that proteins are tiny, incredibly diverse, and perplexingly complicated. To date, researchers have managed to identify and publish the structures of some 168,413 proteins in the Worldwide Protein Database.
Up until recently, the structure of proteins that have been solved are portrayed in still pictures. However, proteins are in constant motion, so the still picture doesn’t reveal the full story.

In order to solve these structures, researchers must visit one of five XFEL facilities that exist worldwide. Opportunities are severely limited given the traffic jam of researchers seeking to schedule time on the facilities.
Concerned about the gridlock, a team of researchers from the Biodesign Institute, led by Wei Liu, a researcher at the Biodesign Center for Applied Structural Discovery, took on the challenge. In a proof-of-concept experiment, Liu and his crew used pink-beam serial crystallography to determine if it offers an effective alternative.
“By leveraging this innovative X-ray diffraction source – the pink beam – we are able to obtain the real-time dynamic structural details of important drug targets,” said Liu. According to Liu, “this finding will accelerate the development of precision medicines to benefit human health.”
The United States Department of Energy Office of Science User Facility recently published "The Future is Pink for Molecular Movies," explaining the team’s experiment in which they, “combined a high-brightness photon source with the latest high-viscosity injection technology to deliver thousands of nano-or microcrystals to the beam.” According to the article, “this approach can generate a complete dataset in a shorter time with a smaller sample volume, providing time-resolved data without damaging radiation exposures.”
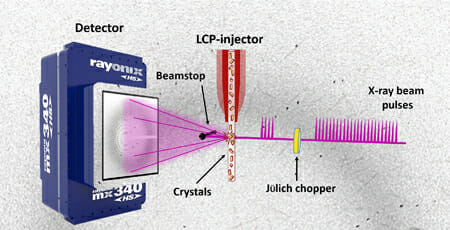
The groundbreaking aspect of this experiment is that it uses a new, less damaging pink beam of light. The pink light allows the researchers to increase the number of photons to a power that is 100 times higher than the monochromatic beams that have been used previously. The team was able to show that they can get complicated shots of the proteins while avoiding the typical damage that occurs when testing the samples in standard light.
Liu and his team worked with collaborators at Argonne National Laboratory, The University of Chicago and the University of Southern California
*adapted from the U.S. Office of Science Article: the Future is pink for molecular movies by Sandy Field